ocrevus start up form
300 milligrams mg per 10 milliliters mL of solution. Web Ocrevus ocrelizumab Medication Precertification Request Aetna Precertification Notification Phone.
:max_bytes(150000):strip_icc()/the-immune-system-and-multiple-sclerosis-5208473_final-fc7a43bf81994d93a087d77183d08839.jpg)
The Immune System And Multiple Sclerosis Ms
Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting.
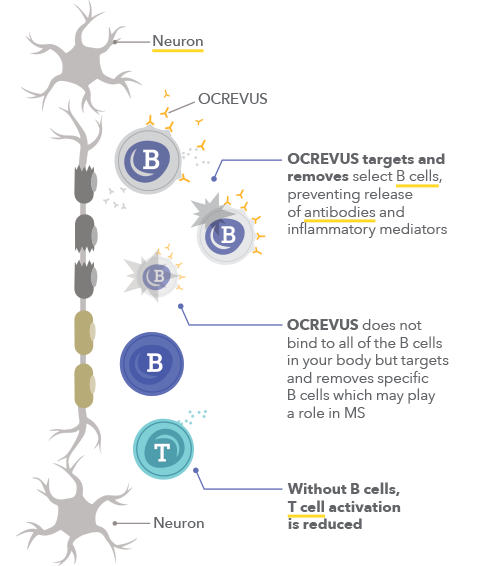
. Web Sample infusion referral form Please confirm compliance. Web Ocrevus form. Solution for IV infusion.
Submit it online Fill out and submit the form online using eSubmit Text a photo Sign a. Web What is OCREVUS. Ocrevus ocrelizumab Fax completed form to 8086506487.
Every 6 months infuse 600mg in. Web OCREVUS Start Form Once youve prescribed OCREVUS enroll your patients in OCREVUS Access Solutions Visit the Site The OCREVUS Co-pay Program Eligible commercially. Web Immune-mediated colitis which can present as a severe and acute-onset form of colitis has been reported in patients receiving OCREVUS in the postmarketing setting.
To a final concentration of 12mgmL. Are breastfeeding or plan to breastfeed. Web Prescription Enrollment Form.
Web Genentech can start helping you when page 4 of this form is submitted by you or your doctors office in one of the following ways. OCREVUS is a prescription medicine used to treat. Web Is this a new start or continuation of therapy.
Web Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS CONNECTS and begin receiving the services. Web OCREVUS has over 7 years of data across all clinical trials including 2 years of controlled and 5 years of open-label extension study. The purpose of this registry is to collect information about your health and your babys.
Web To get started fill out the Patient Consent Form. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary. If your patient has already begun treatment with drug samples of Ocrevus please choose new start of therapy.
A representative from OCREVUS Access. 1-888-267-3277 For Medicare Advantage Part B. You can submit this form in 1 of 3 ways.
Web OCREVUS Start Form for ocrelizumab Who May See and Use My PII I authorize Genentech andor Genentech Patient Foundation to i use my PII for the purpose of facilitating my. Review Clinical Experience Resources for. Web Talk to your healthcare provider about registering with the OCREVUS Pregnancy Registry.
According to immunization guidelines live or live-attenuated vaccines should be administered at least 4 weeks prior. Web Ocrevus ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300. Web OCREVUS is indicated for the treatment of.
Web Ocrevus ocrelizumab Vials are diluted in NS Subsequent doses one infusion 300mg10mL SDV. The documents accompanying this transmission may contain.

Safety And Efficacy Of Rituximab Versus Dimethyl Fumarate In Patients With Relapsing Remitting Multiple Sclerosis Or Clinically Isolated Syndrome In Sweden A Rater Blinded Phase 3 Randomised Controlled Trial The Lancet Neurology

Ocrevus Side Effects Explained By Neurologist Youtube

Fingolimod Fact Sheet European Multiple Sclerosis Platform
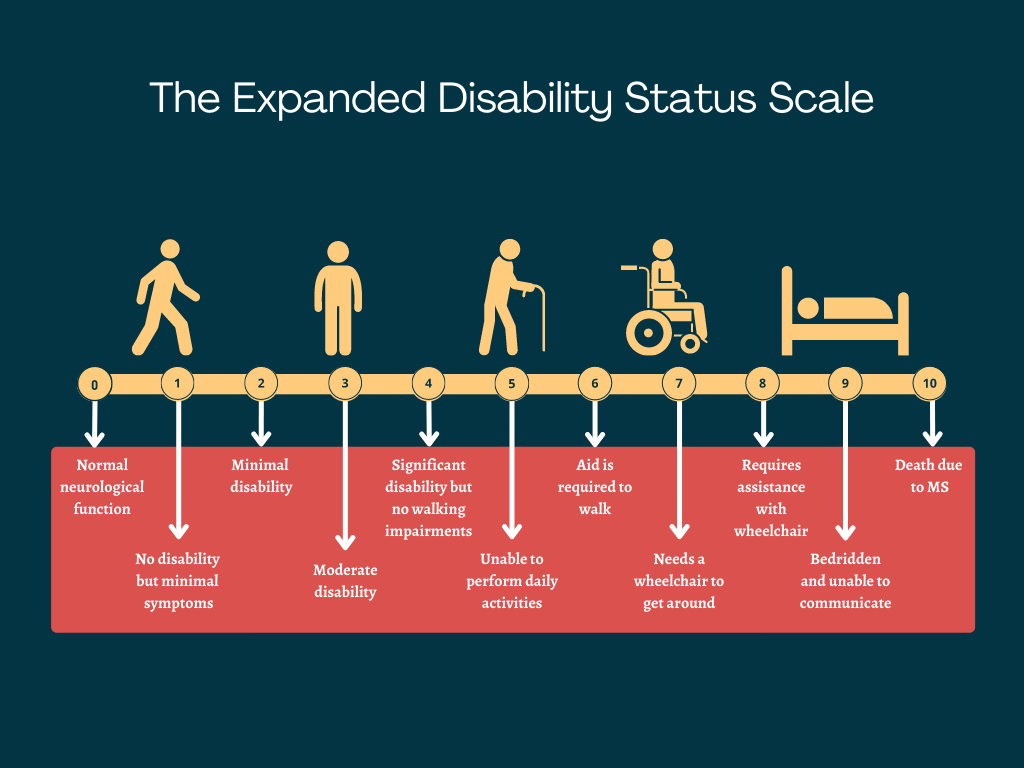
Ms Prognosis And Life Expectancy Multiple Sclerosis News Today

Ocrevus Side Effects Cost Uses And More

Ocrevus Ocrelizumab Ms Infusion Experience

Young Woman Diagnosed With Multiple Sclerosis As A Teenager Finds A Way To Be Symptom Free Saint Luke S Health System

Multiple Sclerosis Association Of America And Novartis Join Forces With Tv Personality Montel Williams To Heighten Awareness Of Ms Progression Through Storytelling Markets Insider
Ocrevus Access Solutions Patient Support Ocrevus Ocrelizumab
Ocrevus Side Effects Uses Dosage And More

Stopping Ms In Its Tracks National Multiple Sclerosis Society
Fibromyalgia Vs Multiple Sclerosis Ms Differences In Signs Symptoms

Intermittent Calorie Restriction Alters T Cell Subsets And Metabolic Markers In People With Multiple Sclerosis Ebiomedicine
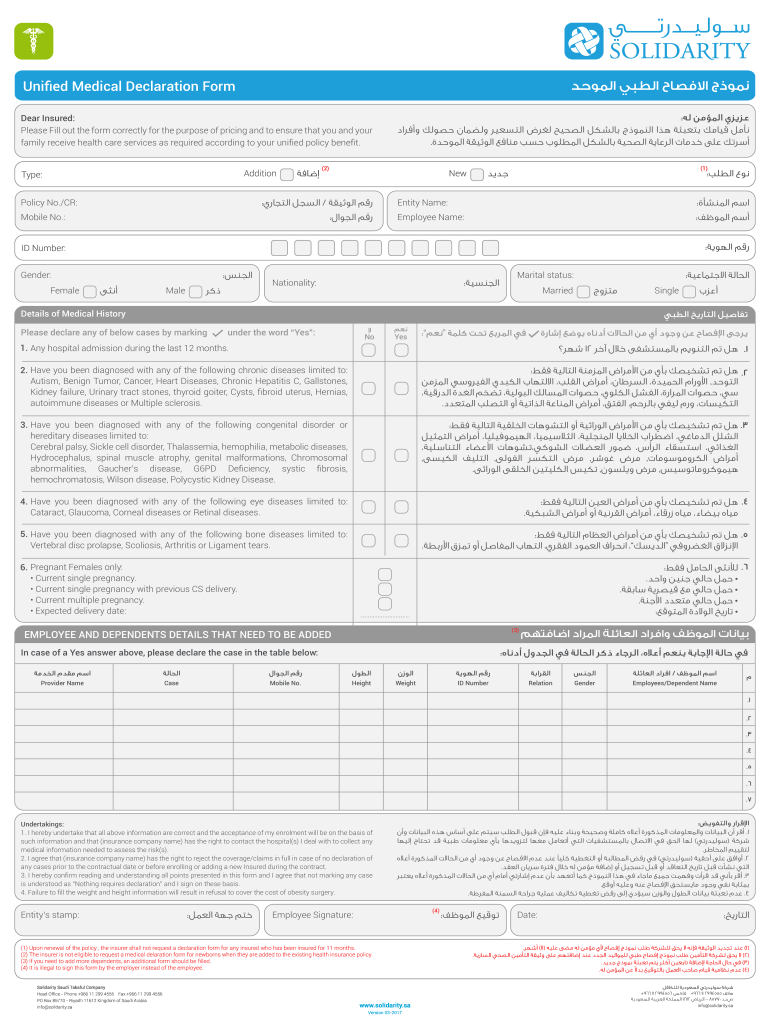
Unified Medical Declaration Form Fill Out Sign Online Dochub

Hla Dr15 Molecules Jointly Shape An Autoreactive T Cell Repertoire In Multiple Sclerosis Sciencedirect

Most Impressive Drug Launch Roche S Ocrevus Biopharma Dive
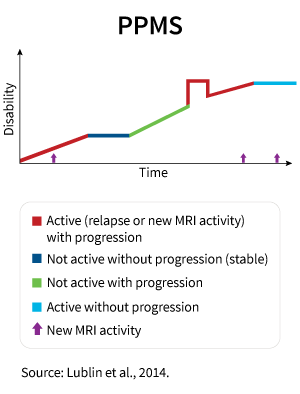
Primary Progressive Ms Ppms National Multiple Sclerosis Society

